Nanoscience will change the way we think about the world
The ubiquity of mineral nanoparticles in natural waters, the atmosphere, and in soils and their intriguing properties provide Earth scientists with another dimension in which to understand our planet.
So states a team of scientists from seven universities in a review article in the March 21, 2008, issue of Science, “Nanominerals, Mineral Nanoparticles, and Earth Chemistry.”
The way minerals influence earth is more complex than previously thought. Physical, chemical, and biological processes on Earth are either influenced or driven by the physical and chemical properties of minerals, of which 4,500 species have been described. Minerals have an enormous range of physical and chemical properties due to a wide range of composition and structure, including particle size.
When the National Science Foundation wanted expert opinion on the important questions that need to be addressed in order to advance the understanding of nanoparticles in the environment, they contacted Michael Hochella Jr. to assemble a group of “cutting-edge young scientists with new ideas,” he said. Hochella, university distinguished professor of geosciences at Virginia Tech, is a pioneer in the field whose research is funded by the National Science Foundation, among others. “While we were together, we thought, why not write our study and submit it to Science,” Hochella said.
“The article looks at the field, where it’s come from, where it’s going, and how it is going to change the way we think about geoscience and the world,” he said. A perspective article is a great challenge to write, considering Science’s limits on length and number of citations, he added.
The authors are Hochella; his former Ph.D. students Steven K. Lower, now a professor in the School of Earth Sciences and School of Environment and Natural Resources at the Ohio State University, and Patricia A. Maurice, now professor of civil engineering and geological sciences and director of the Center for Environmental Sciences and Technology at the University of Notre Dame; along with R. Lee Penn assistant professor of chemistry at the University of Minnesota; Nita Sahai, associate professor of geology and geophysics at the University of Wisconsin-Madison; Donald L. Sparks, chair of plant and soil sciences and professor in three departments at the University of Delaware; and Benjamin S. Twining, assistant professor of chemistry and biochemistry at the University of South Carolina.
Minerals, it is generally agreed, are naturally occurring crystalline substances having a characteristic and defined chemical composition. Each mineral expresses a set of specific physical and chemical properties. In addition, nanominerals have one critical difference. They express a range of physical and chemical properties depending on their size and shape.
“This difference changes our view of the diversity and complexity of minerals and how they influence Earth systems,” Hochella said.
Where nanominerals are located
Nanominerals are widely distributed throughout the atmosphere, oceans, surface and ground waters, and soils, and in most living organisms, and even within proteins.
Oceans may be the principal reservoir, since they cover 70 percent of the Earth’s surface. There, nanominerals can come from processes associated with both living and non-living things, Hochella said. “Every mineral goes through a nanophase stage as it begins to grow. If they begin to grow at many sites, but don't continue to grow much after they form, you will end up with a lot of them and they may persist.”
In addition to growth and weathering, mineral nanoparticles can be generated from mechanical grinding. One of the most interesting and important places where this happens is along earthquake-generating faults in the Earth’s crust, reported by several researchers cited in the review.
There is a distinction between clusters of atoms and nanoparticles, Hochella said. “The difference seems to be that clusters start to approach the size of the smallest nanoparticles, but the atoms in many of these small clusters are not packed very tightly together. They are not dense. The nanoparticles represent a much denser packing of atoms, more like a real mineral, or at least approaching the atomic packing density of a larger mineral.”
The essence of nanoscience is observing, measuring, and understanding the variations of properties and reactivities as a function of size and shape. Structural variations that respond to size change or surface area change may include expansion and contraction of bonds, changes in bond angles, and variations in population and distribution of vacancies and other defects such as steps, kinks, edges, and corners. In the smallest nanoparticles, this results in a redistribution of electronic structure that affects reaction characteristics with the outside world. Measurement of these aspects remains a great challenge and priority for future mineralogists, the authors note.
The size at which properties and reactivities change can be measured and depends upon the mineral, whether it is a metal, semiconductor, or insulator; and on the property being measured, whether optical, mechanical, or electrical.
Chemical interactions also change. For example, seven nanometer hematite -- a common iron oxide mineral -- catalyzes the oxidation of manganese ions (Mn2+) one to two orders of magnitude faster than does a 37-nanometer hematite crystal, resulting in the rapid formation of the manganese oxide minerals that are important heavy metal sorbants in water and soils.
Thermodynamic considerations in the nano-range are just as critical to predicting whether a biogeochemical reaction will occur. In the smallest particles, surface energies can dominate and dictate which structure of a mineral will be stable. Solubility’s of nanophases are also different than their larger counterparts. “But experiments have shown that nanoparticles may or may not be more soluble than larger particles,” Hochella said.
How nanoparticles influence earth chemistry
An example of the impact of nanoparticles is how they nurture ocean-dwelling phytoplankton, which removes carbon dioxide from the atmosphere. Phytoplankton growth is limited by iron availability, the authors report, citing research by J. Wu, E. Boyle, W. Sunda, L.S. Wen, and B.A. Berquist in two articles from 2001 and 2007. Iron in the ocean is composed of nanocolloids, nanominerals, and mineral nanoparticles, which is supplied by rivers, glaciers, and atmospheric deposition. Nanoscale reactions resulting in the formation of phytoplankton biominerals such as calcium carbonate are also important influences on oceanic and global carbon cycling.
Another example is the movement of harmful heavy metals in the Earth’s critical zone. In ongoing research at the Clark Fork River Superfund Complex in Montana, Hochella’s group discovered a nanocrystalline vernadite-like mineral (a manganese oxyhydroxide) involved in the movement of lead, arsenic, copper, and zinc hundred of miles in the river drainage basin. Radionuclides can also be moved, the review reports. Research by A.P. Novikov (2006) at one of the most contaminated nuclear sites in the world, a nuclear waste reprocessing plant in Mayak, Russia, has shown that plutonium has traveled in local groundwater, carried by nanoparticles of less than 15 nanometers.
In the atmosphere, nanoparticles impact heating and cooling. The characteristics of atmospheric nanoparticles are critical and are now being studied by a large number of scientists. One observation is that such particles act as water drop growth centers, which is critical to cloud formation. The size and density of droplets dictates solar radiation scattering ability and cloud longevity, which influence average global temperatures.
The authors conclude that “The biogeochemical and ecological impacts of natural and synthetic nanomaterials is one of the fastest growing areas of research today, with not only vital scientific, but also large environmental, economic, and political consequences”.
- Learn more about the Hochella group.
- Read "Geoscientist solves living mystery, works to help environment."
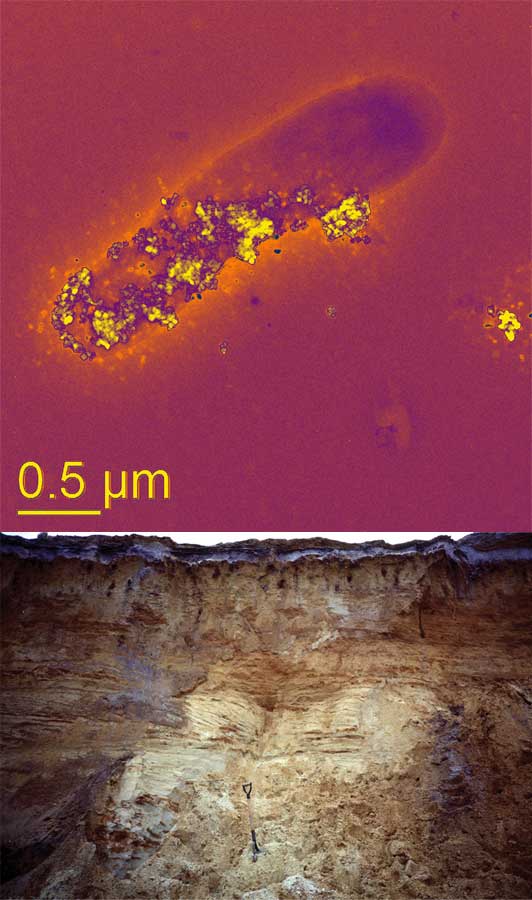
PHOTO INFORMATION: (Top image) A bacterial cell is respirating using mineral nanoparticles, since there is no oxygen around. Photo by Saumyaditya Bose, a 2006 Ph.D. graduate of geosciences (Virginia Tech).
(Bottom image) A soil profile in the New Jersey Pine Barrens, where many nano-geo processes take place. Courtesy of Patricia Maurice (Notre Dame).



.jpg.transform/m-medium/image.jpg)