Like cars on a highway: Researchers dig deeper into how migrating cells interact in the body
By offering a microscopic "tightrope" to cells, Virginia Tech and Johns Hopkins University researchers have brought new insights to the way migrating cells interact in the body. The researchers changed their testing environment for observing cell-cell interaction to more closely mirror the body, resulting in new observations of cells interacting like cars on a highway — pairing up, speeding up, and passing one another.
Understanding the ways migrating cells react to one another is essential to predicting how cells change and evolve and how they react in applications, such as wound healing and drug delivery. In a study published in the Proceedings of the National Academy of Sciences, a team formed by Mechanical Engineering Associate Professor Amrinder Nain, graduate researchers Jugroop Singh and Aldwin Pagulayan, and Johns Hopkins Assistant Professor Brian Camley, pivoted from traditional testing methods to more accurately observe how moving cells behave when they encounter one another.
Cells have polarity, a north-south orientation, like common magnets. Polarizing helps a cell orient itself correctly with other cells and also establish a leading and trailing edge – the front and the back ends of the cell in motion. As cells move and divide, their polarities change and shift, a product of the motion of molecules within them. Each cell’s motion is controlled by protrusions – tentacles that pull each cell along at the leading edge, controlling its motion – and when migrating cells collide, they tend to repel one another, causing their protrusions to contract inward. Cells form new protrusions away from the collision to change direction and go to new places.
This behavior of changing direction after collisions is called contact inhibition of locomotion, and it brings about a change in the cell’s poles. After a collision, the trailing edge becomes the leading edge, thus reversing the migration direction.
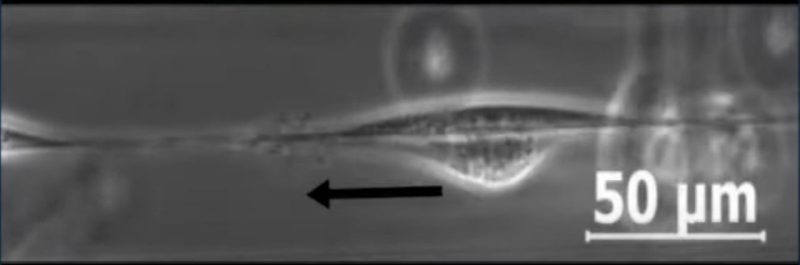
Since the 1950s, contact inhibition of locomotion has been observed by placing cells in a flat environment, such as a petri dish. This is unlike the setting of a body, however, where cells move along networks of fibers. To bring their observations closer to the natural environment, the team introduced cells onto a single fiber, a kind of cellular "tightrope." In that setting, they found that cell-cell interaction was entirely different than that of a flat surface. Whereas cells collide on a flat surface, they become far more civil when walking along a fiber. When sharing the tightrope, given nowhere to go, cells tended to move past one another.
Of course, a body is made of many fibers, not just one. To further investigate cellular behavior in its natural environment, the team introduced a second, parallel fiber. Cellular behavior changed again: instead of moving past one another, cells would stick together, moving in pairs with one of the cells changing its poles.
Another change occurred when cells divided. After a new cell was formed from a division, called a “daughter cell,” they tended to walk past other cells more often in both configurations. The researchers found that daughter cells moved with increased speed, and this was likely a contributor to their ability to move past more often.
The team was able to recreate these behaviors with a simple model that assumed cells crawled along the fibers and reoriented when they came in contact with another cell's front, and that daughter cells moved faster.
"This cocktail of mechanical engineering, cell biology, physics, and computational modeling reveals cell behaviors not known before," said Nain. "Cells and their environments are complex and constantly changing. Our collaborative work adds a twist to the knowledge of contact inhibition of locomotion, first discovered in the 1950s.”
According to Camley, knowing this information furthers understanding into why some drugs work differently in a test on a petri dish than in an animal. The difference in flat-surface behavior versus fiber provides insights that could mean the difference between a impacting a cell with a drug or missing it, given a more holistic view of cellular responses.
"Instead of changing our view about the underlying biology, this shows how physical changes in the cell's environment can alter cell-cell interactions," said Camley. "Scientists often want to figure out how drugs can alter cell-cell interactions — our results show that it's important to study these in as natural an environment as possible because environment plays a huge role."
— Written by Alex Parrish




